Members
Masashi Aoki (Neurology)

Dr. Aoki graduated Tohoku University School of Medicine (MD) and was given PhD degree from the same university. For 1996-1998, he worked abroad as a postdoctoral fellow and Instructor in Neurology in Massachusetts General Hospital under supervisory of Dr. Robert H Brown Jr. and investigated the genetics for amyotrophic lateral sclerosis and muscular dystrophy. He has been recognized as one of the distinguished researchers of neurological disorders. He is known as a recipient of ALS Foundation, Japan ALS Association in 1995, the Nakabayashi Trust for ALS Research in 1999 and the award from Japan Intractable Disease Research Foundation in 2001.
- Introduction of Research
-
Development of motor neuron restorative therapy in amyotrophic lateral sclerosis using hepatocyte growth factor
Amyotrophic lateral sclerosis (ALS) is an adult onset neurodegenerative disorder characterized by the death of upper and lower motor neurons. Mutations in Cu/Zn superoxide dismutase (SOD1) have been linked to some familial cases of ALS1). In familial ALS kinders with mutations in the SOD1 gene, the age of onset of weakness varies greatly but the duration of illness appears to be characteristic to each mutation. For example, in patients with the L84V mutation, the average life expectancy is less than 1.5 year after the onset of symptoms, whereas patients harboring the H46R mutation have an average life expectancy of 18 years after the disease onset. In view of the evidence supporting the idea that familial ALS variants of SOD1 enzymes acquire toxic properties, the variations in the duration of illness in the different kinders might arise because each mutation imparts different degrees of toxicity to the mutant protein2).
We developed rats that express a human SOD1 transgene with two different ALS-associated mutations (G93A and H46R) develop striking motor neuron degeneration and paralysis. The larger size of this rat model as compared with the ALS mice will facilitate studies involving manipulations of spinal fluid (implantation of intrathecal catheters for chronic therapeutic studies; CSF sampling) and spinal cord (e.g., direct administration of viral- and cell-mediated therapies) (Fig 1) 4).
Hepatocyte growth factor (HGF) is one of the most potent survival-promoting factors for motor neurons. To examine its both protective effect on motor neurons and therapeutic potential, we administered human recombinant HGF (hrHGF) by continuous intrathecal delivery to G93A transgenic (Tg) rats at onset of paralysis for 4 weeks. Intrathecal administration of hrHGF attenuates motor neuron degeneration and prolonged the duration of the disease by 63 % (Fig.2, 3) 6). Our results indicated the therapeutic efficacy of continuous intrathecal administration of hrHGF in Tg rats. The results should prompt further clinical trials in ALS using continuous intrathecal administration of hrHGF. We are making efforts in evaluating several adverse effects of the hrHGF treatment on primates using marmosets on the way to clinical trials of HGF for ALS patients.
Positional cloning of the gene for Miyoshi myopathy and limb-girdle muscular dystrophy
Miyoshi myopathy (MM) is an autosomal recessive distal muscular dystrophy characterized by mid-to late childhood or early adulthood onset, with preferential involvement of the calf muscles and highly elevated levels of the enzyme serum creatine kinase (CK). In 1998, we identified that the dysferlin gene is mutated in MM3). It has 55 exons and 6,243-bp nucleotides in an open reading frame predicted to encode 2,080 amino acids. This gene is also mutated in families with limb girdle muscular dystrophy 2B3). We reported the genotype-phenotype correlations in Japanese patients with MM5).
Figure 1.
Intrathecal administration of HGF to transgenic (Tg) rats protects against neurodegeneration. Some experimental manipulations are difficult in Tg mice because of size limitations. However, this Tg rat model allows routine implantation of infusion pumps for intrathecal drug delivery. This route of administration bypasses the blood-brain barrier, allowing rapid access to potential binding sites for the test compound in the spinal cord.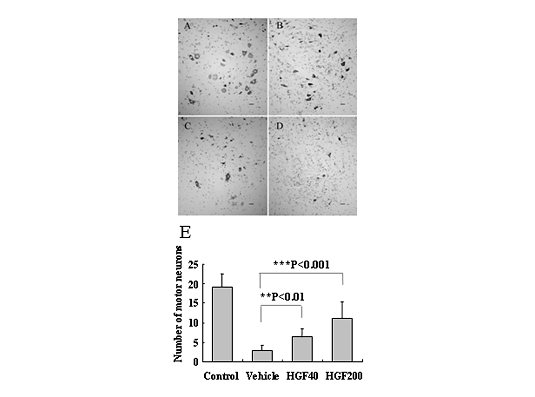
Figure 2.
Intrathecal administration of HGF to G93A Tg rats from 100 days old (the age at which pathological changes of the spinal cord appear but animals show no clinical weakness) showed a protective effect against motor neuron death6). (A-D) Histological evaluation of the anterior horn with Nissl staining at 130 days old. The anterior horn of the lumbar cord of non-Tg rats (A), 200 μg human recombinant HGF (hrHGF) treated (B), 40 μg hrHGF-treated (C), and vehicle-treated G93A Tg rats (D) (scale bar, 40 μm). (E) Quantitative morphometric evaluation of surviving motor neurons of the fifth lumbar anterior horn at 130 days old. We counted neurons that were >40 μm in diameter. Significantly larger numbers of motor neurons survived in hrHGF-treated G93A Tg rats (P<0.01 and P<0.001, 40 μg and 200 μg hrHGF, respectively) as compared with vehicle-treated G93A Tg rats.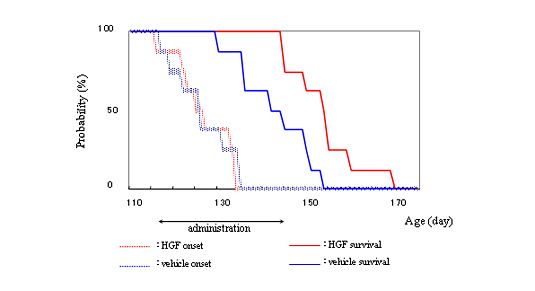
Figure 3.
Intrathecal administration of HGF from 115 days old (just before disease onset) retarded disease progression6). Survival periods were 143.25±17.0 d in the vehicle-treated group (solid blue line) and 154.3±16.4 d in the 200 μg hrHGF-treated group (solid red line). Survival of hrHGF-treated animals was significantly extended (P=0.0135), although there were no significant differences in onset (dotted lines, P=0.6346). - Articles
-
- Aoki M, Ogasawara M, Matsubara Y, Narisawa K, Nakamura S, Itoyama Y, Abe, K. Mild ALS in Japan associated with novel SOD mutation, Nature Genet (1993) 5: 323-4
- Aoki M, Abe K, Houi K, Ogasawara M, Matsubara Y, Kobayashi T, Mochio S, Narisawa K, Itoyama Y Variance of the age at onset in a Japanese family with amyotrophic lateral sclerosis associated with a novel Cu/Zn superoxide dismutase mutation, Ann Neurol (1995) 37: 676-9
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, J. Urtizberea A, Hentati F, Mongi Ben Hamida M, Bohlega S, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown, RH Jr. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathyand limb girdle muscular dystrophy, Nature Genet (1998) 20: 31-6
- Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH Jr, Itoyama Y. Rats expressing human cytosolic Cu/Zn superoxide dismutase transgenes with amyotrophic lateral sclerosis-associated mutations develop motor neuron disease. J Neurosci (2001) 21: 9246-54
- Takahashi T, Aoki M, Tateyama M, Kondo E, Mizuno T, Onodera Y, Takano R, Kawai H, Kamakura K, Mochizuki H, Shizuka-Ikeda M, Nakagawa M, Yoshida Y, Akanuma J, Hoshino K, Saito H, Nishizawa M, Kato S, Saito K, T, Miyachi T, Yamashita H, Kawai M, Matsumura T, Kuzuhara S, Ibi T, Sahashi K, Nakai H, Kohnosu T, Nonaka I, Arahata K, Brown RH Jr, Saito H, Itoyama Y. Dysferlin mutations in Japanese Miyoshi myopathy: Relationship to phenotype, Neurology (2003) 60: 1799-1804
- Ishigaki A, Aoki M, Nagai M, Warita H, Kato S, Kato M, Nakamura T, Funakoshi H, Itoyama Y, Intrathecal delivery of HGF from the ALS onset suppresses disease progression in a rat ALS model, J Neuropathol Exp Neurol (2007) 66: 1037-44