Members
Noriko Osumi (Developmental Neuroscience)

Prof. Osumi has graduated Tokyo Medical and Dental University, been given PhD thesis from the same university, and now is a professor of Tohoku University School of Medicine since 1998. She is appointed in various governmental committees such as ethical issues, grant system development, and career paths for young scientists, and also chosen as a youngest member of Japanese Council Japan since 2005. Her research interest covers broad areas such as pre- and postnatal development of the brain and craniofacial region, and behavior of animals as models of psychiatric diseases. More specifically, she is recently eager to understand regulatory mechanisms of neurogenesis and maintenance of neural stem cells at cellular and molecular levels both in embryonic and postnatal stages. Manipulating embryos and imaging brain cells are expertise of her lab. She has translated two books into Japanese: Essential Developmental Biology by Jonathan Slack and The Birth of the Mind by Gary Marcus. She is a representative of CREST project (2005-2009) supported by JST and Global COE project (2007-2011) supported by MEXT.
- Introduction of Research
-
In order to achieve various higher functions of the brain, several developmental processes have to be accomplished. For example, various kinds of neurons have to be generated and distributed in accurate numbers and in precise positions, and proper neural circuits have to be established among an enormous numbers of neurons. In addition to these neurons, a huge number of glial cells (astrocytes, oligodendrocytes, and microglia) are also located within the entire central nervous system (CNS), where the astroytes interact with blood vessels to intake oxygen and nutrients and to transfer them to the neurons, oligodendrocytes myelinate neuronal axons thereby increasing speed of neuronal transmission, and microglia work in healing inflammation and wound. Most of the processes in the brain formation during embryonic periods are governed by genetic programs, yet further refinement and modification of the CNS continue postnatally. During the initial process of CNS formation when the neural tube is just closed, the region called the neural crest is established at the interface between the neural epithelium and surface ectoderm. Neural crest-derived cells differentiate multiply to make not only neurons and glia in the peripheral nervous system (PNS), but also bone, cartilage, smooth muscle and pericytes of the blood vessels in the craniofacial region. Our lab is thus working to better understand mechanisms for development of the CNS and PNS at molecular and cellular levels. Particularly, we are interested in initial brain regionalization, embryonic and adult neurogenesis (i.e., proliferation and differentiation of neural stem cells), and mechanisms for establishment and differentiation of the neural crest. Our recent curiosity in neurogenesis further includes relationship between states of neurogenesis and mental diseases because the neurogenesis is important not only in brain formation but possibly in homeostasis of brain functions.
Above studies require various experimental systems, which we are actively developing. For example, we have established a unique system to transfer certain genes directly into the developing brain primordium by combining mammalian whole embryo culture and electroporation. This technique is very quick and easy compared with one using virus vectors, and has advantage in precisely transferring genes into certain regions of the brain primordium. For manipulating embryos at later stages, we perform in utero operation together with electroporation. Time-lapse imaging techniques are also refined to observe embryonic neural stem cells in conditions better mimicking in vivo situation. Behavior analyses of rodent models have been done in regards with neurogenesis within the brain development and aging and mental diseases.
Various kinds of environmental factors influence on development and maintenance of the brain. We are particularly focusing on nutrients, and analyzing effects of polyunsatulated fatty acids (e.g., DHA and arachidonic acid) on neurogenesis in animal models and cultured cells. Since exercise promotes neurogenesis in rodents possibly via enhancing blood flow, we are trying to increase neurogenesis with treating mice with antihypertensive drugs. These studies may contribute therapeutic development for prevention and treatment of mental diseases such as depression and schizophrenia that may relate with impaired neurogenesis.
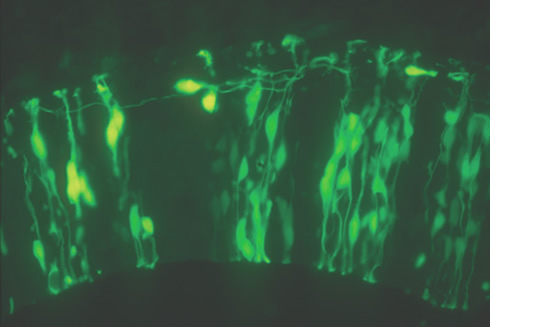
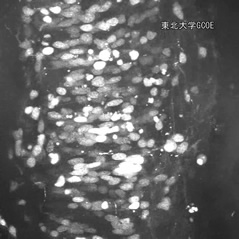
Fig. 1. Morphology of neuroepithelial cells and differentiated neurons in the developing rat hindbrain. Neuroepithalial cells are highly polarized cells with long apical and basal processes, which attach to the lumen of the ventricle and to the pial surface, respectively.
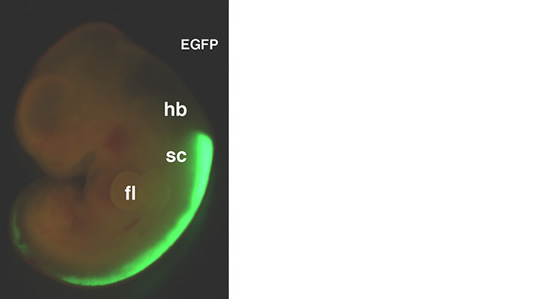
Fig. 2. Transfer of exogenous genes into cultured rat embryo Expression vector of enhanced green fluorescent protein (EGFP) was introduced into neuroepithelial cells in the rat spinal cord. 24 hours later, the expression of EGFP protein is detected by fluorescent microscopy.
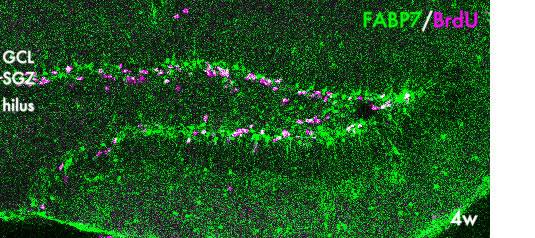
Fig. 3. Neural stem cells in the postnatal hippocampus. Proliferating neural stem/progenitor cells are labeled with BrdU (magenta) and express FABP7, a fatty acid binding protein. GCL:granule cell layer; SGZ, subgranular zone
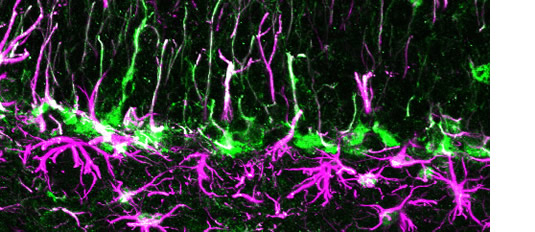
Fig. 4. Neural stem cells and astrocytes in the postnatal hippocampus. The localization of FABP7+/GFAP+ cells in the subgranular zone of the mouse hippocampal dentate gyrus at 4 weeks. These cells are considered to be nueral stem cells and astrocytes.
Movie1. Visualization of interkinetic nuclear movement by time-lapse imaging Expression vector of EGFP-Histone fusion protein gene was introduced into the rat spinal cord by electroporation, and slice was prepared from the electroporated spinal cord. Time-lapse imaging was performed with interval of 5 minutes by confocal laser scanning microscopy. Nuclei of neuroepithelial cells that migrate to apical side are visualized with EGFP-Histone fusion protein. Left side corresponds to the apical side of the neoeputlium.
- Articles
-
- Matsuo, T.*, Osumi-Yamashita N.*, Noji, S., Ohuchi, H., Koyama, E., Myokai, F., Matsuo, N., Taniguchi, S., Doi, H., Iseki, S., Ninomiya, Y., Fujiwara, M., Watnabe, T., & Eto, K.: A mutation of the Pax-6 gene in rat "small eye" was associated with migration defect of midbrain crest cells. Nature Genet. 3, 299-304, 1993
- Osumi, N., Hirota, A., Ohuchi, H., Nakafuku, M., Iimura, T., Kuratani, S., Fujiwara, M., Noji, S., & Eto, K.: Pax-6 is involved in specification of the hindbrain motor neuron subtype. Development 124, 2961-2972, 1997
- Inoue, T., Tanaka, T., Takeichi, M., Chisaka, O., Nakamura, S., & Osumi, N.: The role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development 128, 561-569, 2001
- Nomura, T., and Osumi, N.: Misrouting of mitral cells in Pax6/Small eye rat telencephalon. Development 131, 787-796, 2004
- Arai, Y., Funatsu, N., Numayama-Tsuruta, K., Nomura, T., Nakamura, S., & Osumi, N.: The role of Fabp7, a downstream gene of Pax6, in maintenance of neuroepithelial cells during early cortical development. J. Neurosci. 25, 9752-9761, 2005
- Takahashi, M. & Osumi, N.: Pax6 regulates specification of ventral neuron subtypes in the hindbrain by establishing progenitor domains. Development 129, 1327-1338, 2002
- Tsunekawa, Y., Britto, J.M., Takahashi, M., Polleux, F., Tan, S-S., and Osumi, N.: Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. EMBO J, online published, 2012