Members
Daisuke Yamamoto (Behavior Genetics)

Daisuke Yamamoto is a full professor of Neurogenetics at Tohoku University Graduate School of Life Sciences, Sendai, Japan. He majored in Applied Entomology and Zoology (1976) at Tokyo University of Agriculture and Technology, Japan, and after that, he did his graduate studies in Neurophysiology in Mitsubishi-Kasei Institute of Life Sciences, Machida, Tokyo, and received his D. Sc. (equivalent to Ph.D.) in 1981 at Hokkaido University Graduate School of Science. In 1980, he was employed as Junior Staff Scientist in Mitsubishi-Kasei Institute of Life Sciences, and worked there until 1999 (promoted to Group Leader in 1988). He had a postdoctoral training at Northwestern University Medical School, Chicago, USA, from 1981 to 1983. He was appointed to be Director of ERATO Yamamoto Behavior Genes Project from 1994 to 1999. Yamamoto was on the faculty of Waseda University as a full professor in 1999. He moved to Tohoku University in 2005. Yamamoto explores our long-standing question of why females and males think and behave so differently. His favorite material is a Drosophila mutant fruitless, males of which display homosexual courtship. He was the first to clone the gene fruitless (1996). Yamamoto is also prolific in his writing on science.
- Introduction of Research
-
Why females and males behave differently is a fundamental question for us. Biologists postulate that the sexual dimorphism in the brain underlies such gender differences in behavior, yet little evidence has been obtained. Drosophila offers a handy system for genetic dissection of complex behaviors. One of the most salient examples of sexually dimorphic behaviors in Drosophila is courtship behavior. When a male fly encounters a female, he immediately initiates courtship in most cases, whereas the male-male encounters provoke aggression under certain conditions. The male fly could also commence courting another male but he stops courting soon after, often switching to aggression. Such a choice between behavioral repertoires correlated with the differences in the social context must result in a switching of motor centers to be activated, and this decision-making is likely controlled by an executive neural center that is situated on a higher rung of the neural hierarchy. To explore the neural basis for decision-making, we have to identify these neural centers individually in the brain.
We will use the MARCM (Mosaic Analysis with Repressive Cell Markers) method to label a few clonal cells in the brain, to manipulate them, or to record their activity. In MARCM, the Gal4 action is repressed by the presence of Gal80 in most cells in the body, except for some clonal cells in which the Gal80-coding transgene has been recombined out during development. The cells derived from these Gal80-free clones alone express Gal4 in the animal. Gal4 is then used to drive expression of reporters, activators or inactivators of neurons. In the proposed research, we use MARCM to study physiology and anatomy of a subset of fruitless-expressing cells that potentially contribute to sexual dimorphisms of behavior.
The fruitless gene was originally identified by its remarkable phenotype of its mutants: mutant males court both males and females, yet without copulating. In some of other strains that are also mutant for the fruitless gene, males court males but not females. Therefore, the fruitless gene seems to function as a critical switch in behavioral choice in the sexual context. In fact, the fruitless gene is proposed to be a master control gene in organizing the brain centers for sexual behavior, for its ability to cause an 。ネinversion。ノ of the gender role, when male-specific forms of its transcripts are expressed in females by replacing the genomic fruitless gene with an engineered one.
We will identify uniquely the fruitless-expressing neurons by labeling individually their entire structures including dendrites and axons in addition to the cell bodies by means of the MARCM method. Based on the projection and dendritic branching patterns of fruitless-expressing neurons revealed by single cell labeling with this method, we should be able to distinguish groups of neurons that are functionally distinct. The neuronal clusters we define here are presumably not just the cell groups of which somata are located close proximity to each other, but represent functional units, as the members of a group share projection patterns and dendritic fields. If this is the case, we may be able to assign a specific function to each neuronal cluster. For instance, a particular cluster of fruitless-expressing neurons could play an executive role in initiating male-typical courtship behavior. We will generate small clonal patches of neurons that are masculinized in otherwise female individuals, on the assumption that a fraction of such females displays male-like courtship as a result of sexual transformation of a cell cluster that functions as the neural center to trigger the male-type sexual behavior.
This rather straightforward approach using Drosophila genetics will allow us to identify executive neurons governing behavioral choices in the sexual context. Do such executive neurons for sex-specific behavior exist in humans, as well? This remains to be answered.
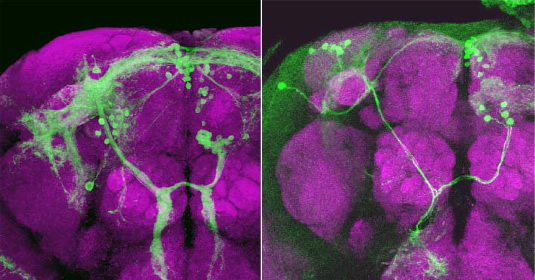
Figure: Sexually dimorphic neuronal cluster, mAL, that express fruitless. mAL is composed of 30 cells in males and 5 cells in females. The neurite projection and dendritic arborization of mAL are also sexually dimorphic.
- Articles
-
- Ito, H., Sato, K., Koganezawa, M., Ote, M., Matsumoto, K., Hama, C., and Yamamoto, D. Fruitless cooperates with two antagonistic chromatin factors to establish single-neuron sexual dimorphism. Cell (2012), in press.
- Kohatsu, S., Konganezawa, M., and Yamamoto, D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 69, 498-508. 2011.
- Nojima, T., Kimura, K-I., Koganezawa, M., and Yamamoto, D. Neuronal synaptic outputs determine the sexual fate of postsynaptic targets. Curr. Biol. 20, 836-840. 2010.
- Koganezawa, M., Haba, D., Matsuo, T., and Yamamoto, D. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Curr. Biol. 20, 1-8. 2010.
- Kimura, K-I., Hachiya, T., Koganezawa, M., Tazawa, T., and Yamamoto, D. Fruitless and Doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59, 759-769. 2008.
- Kimura, K-I., Ote, M., Tazawa, T., and Yamamoto, D. fruitless specified sexually dimorphic neural circuitry in the Drosophila brain. Nature 438, 229-233. 2005.