Members
Hiromu Yawo (Neuron Network)

Dr. Hiromu Yawo graduated Kyoto University Graduate School of Medicine in 1981, being given PhD under the thesis How a nerve fiber repairs its cut end: involvement of phospholipase A2. He thereafter collaborated with Prof. Motoy Kuno in Kyoto University as an instructor of physiology. In 1985 he received an award from the Center for Cellular and Molecular Neurobiology in Washington University School of Medicine in St. Louis, USA (McDonnell Research Fellowship). Under collaboration with Prof. Dale Purves he started studies on the neuronal network plasticity. In 1995 he became a professor of Tohoku University School of Medicine. He has been a professor of Tohoku University Graduate School of Life Sciences since 2001. He organized a research team investigating Presynaptic mechanisms of learning and memory as a Director of the Program of Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Agency (JST) from 1999 to 2005. His major aim of research is to understand the molecular and cellular mechanisms underlying the neuronal network plasticity.
- Introduction of Research
-
The brain consists of many types of neurons which make a complex network. This idea was first proposed by Ramon y Cajal more than 100 years ago. However, it is still unresolved how the network activities are integrated into the brain’s function, the mind. Using genetic engineering techniques we have developed new optical recording methods which visualizes the network activities (Araki et al. 2005). We plan to manipulate activities of individual neurons by light as well as to record them optically, and to reveal how they are integrated in the network. The neuronal network is also dynamically regulated by its environment as well as by its activity itself. We will investigate the cellular and molecular mechanisms regulating the network dynamics.
In the neuronal network of the central nervous system (CNS) the synaptic transmission is regulated by the vesicle exocytosis and endocytosis. This vesicular dynamics is associated with the changes of intra-vesicular pH and can be visualized by the fluorescence of synaptopHluorin (SpH), a pH-sensitive GFP fused to the lumenal aspect of VAMP-2. We have generated several SpH transgenic mouse lines in which the SpH expression is regulated by the neuron specific Thy-1.2 promoter or Cre/loxP recombination system. In one of them SpH was specifically expressed in the mossy fiber (MF) terminals of the hippocampus. Recently we found that there are distinct two vesicle pools, the resting pool which is resistant to exocytosis, and the releasable pool and that the fidelity of synaptic transmission is ensured by the rapid supply from the reserve subpopulation of releasable pool (Suyama et al. 2007).
During phototaxic and photophobic movements of unicellular green algae, light is perceived by archaeal-type rhodopsins which are localized in small regions of the plasma membrane, called eyespots. Two rhodopsins were isolated from Chlamydomonas reinhardii, channelrhodopsin (ChR) 1 and 2. ChR2 has a peak light absorbance at 460 nm and forms a non-selective cation channel, the gating of which is triggered by the photoisomerization of the all-trans retinal to 13-cis configuration. We expressed ChR2 exogenously in the hippocampal neurons of a living mouse (Ishizuka et al. 2006). A brief illumination by a blue LED light depolarized these neurons over threshold to evoke action potential which is phase-locked with the light pulses.
With its high resolution in space and time, its large dynamic range and its convenience this photostimulation method would fulfill all the requirements for artificial stimulation of neurons, namely generality, speed, localization and parallelism. Since ChRs and their derivatives are relatively small and encoded in a single gene, they could be expressed in a specific subset of neurons under regulations of cell-type-specific promoters. It would, thus, open many potential applications (optogenetics), for both in vitro and in vivo studies of neuronal network, artificial manipulations of neuronal activity for the development of informational modules and possibly the development of non-invasive therapeutic instruments of bypassing interrupted neuronal connections.
New neurons are continuously generated in the hippocampus at the subgranular zone of the dentate granule cell layer throughout life. Although the neurogenic processes have been extensively studied using animal model systems in vivo, it appears to be difficult to identify a single progenitor cell and to track its fate for a certain period. To solve this, we used the organotypic slice culture of hippocampus as an ex vivo model preparation (Kamada et al., 2004). The slice culture system enabled us to track a single progenitor cell and its descendents as a lineage in the postnatal hippocampus (Yokose et al., 2011).
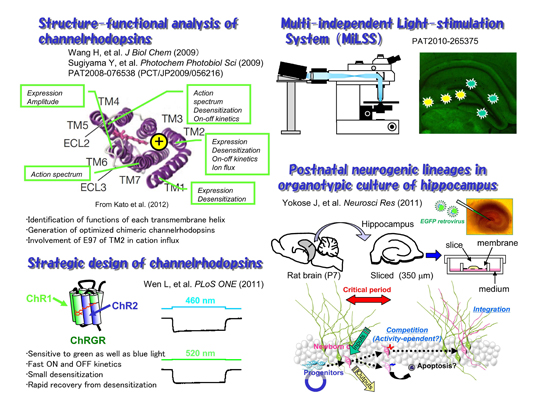
[Figure 1]
Our structure-functional analysis of ChRs suggested that each transmembrane helix has specific function. The glutamate residues in the TM2 are involved in the cation influx. These findings enable us to design new channelrhodopsins optimized for applications. One of them, channelrhodopsin-green receiver (ChRGR) was sensitive to green as well as blue light, has fast ON and OFF kinetics with small desensitization, and showed rapid recovery from desensitization. We also developed an optical system using commercial projectors (MiLSS). It enables one to photostimulate multiple sites in parallel with different temporal patterns and wavelengths. The survival of newborn neurons should be dependent on the activity which would be optically manipulated.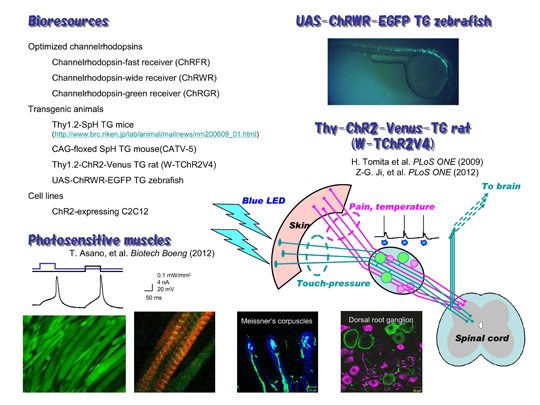
[Figure 2]
We are distributing several bioresources to many laboratories in the world. The chimeric ChRs are powerful tools for optogenetics. We have generated a transgenic rat in which ChR2 was expressed under regulation of thy-1.2 promoter (Thy1.2-ChR2-Venus TG rat). We found that ChR2 was expressed in the touch-sensing neurons and their peripheral nerve endings but not in the pain-sensing neurons in the dorsal root ganglion (DRG). As a result, the rat acquired a supersense that it senses light with its skin as touch-pressure. We also generated transgenic zebrafish expressing channelrhodopsin-wide receiver (ChRWR), one of chimeric proteins of ChR1 and 2, as a conjugate of EGFP under the regulation of UAS promoter (UAS:ChRWR-EGFP). When crossed with any Gal4 line, ChRWR-EGFP would be selectively expressed in the cells expressing Gal4. We made a cloned ChR2-expressing C2C12 myoblasts, which fused with each other to form myotubes. The maturated myotubes became contractile upon illumination with blue light. - Articles
-
- Wang H, Sugiyama Y, Hikima T, Sugano E, Tomita H, Takahashi T, Ishizuka T, Yawo H (2009) Molecular determinants differentiating photocurrent properties of two channelrhodopsins from Chlamydomonas. Journal of Biological Chemistry 284(9): 5685-5696
- Sugiyama Y, Wang H, Hikima T, Sato M, Kuroda J, Takahashi T, Ishizuka T, Yawo H (2009) Photocurrent attenuation by a single polar-to-nonpolar point mutation of channelrhodopsin-2. Photochemical and Photobiological Sciences 8(3): 328-336
- Wen L, Wang H, Tanimoto S, Egawa R, Matsuzaka Y, Mushiake H, Ishizuka T, Yawo H (2010) Opto-current-clamp actuation of cortical neurons using a strategically designed channelrhodopsin. PLoS ONE 5(9): e12893
- Yokose J, Ishizuka T, Yoshida T, Aoki J, Koyanagi Y, Yawo H (2011) Lineage analysis of newly-generated neurons in organotypic culture of hippocampus. Neuroscience Research 69(3): 223-233
- Asano T, Ishizuka T, Yawo H (2012) Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnology and Bioengineering 109(1):199-204.
- Ji Z-G, Ito S, Honjo T, Ohta H, Ishizuka T, Fukazawa Y, Yawo H (2012) Light-evoked somatosensory perception of transgenic rats which express channelrhodopsin-2 in dorsal root ganglion cells. PLoS ONE (in press) DOI: 10.1371/journal.pone.0032699
