医科学専攻
- Master's Courses
修士課程
Investigative Pathology病態病理学
STAFF
Professor
-
Furukawa, ToruProfessor. 古川 徹 教授

Other Faculty / Staff
-
Murakami, Keigo
Lect. 村上 圭吾 講師 -
Hirose, Katsuya
Assistant Prof. 廣瀨 勝也 助教
CONTACT
TEL:+81-22-717-8149
E-MAIL:toru.furukawa.e2*tohoku.ac.jp
(「*」を「@」に変換してください)
OUTLINE
We carried out research of intractable diseases with employing advanced molecular pathobiological techniques and genome analysis. Selected topics of our recent research are as follows:
1. We discovered that the epigenetic silencing of DUSP6 contributes to the constitutive activation of ERK/MAPK in pancreatic cancer. The active ERK promotes expression of numerous genes including AURKA and SON, which are useful molecular targets to attenuate proliferation of pancreatic cancer.
2. We discovered that Intraductal papillary mucinous neoplasm of the pancreas (IPMN) harbor frequent gain-of-function mutations in GNAS. We demonstrated that pancreas-specific expression of the mutant GNAS along with mutated Kras causes to develop IPMN in a genetically engineered mouse model.
3. We developed a system for testing precision medicine in intractable cancers including using patients’-derived organoids and their genome analysis.
当分野では難治性疾患を対象に、最先端のゲノムワイドな分子解析を取り入れた次世代の病理学研究を推進している。
1. 膵臓癌では変異KRASを含む活性化信号にERK/MAPK特異的脱リン酸化酵素であるDUSP6の不活化が相まることでERK/MAPKが恒常的に活性していることを見出し、活性化ERK/MAPKにより誘導される遺伝子のゲノムワイドスクリーニングで膵癌の悪性形質を担い、かつ、標的化により診断や治療に応用できる分子を同定した。
2. 膵管内乳頭粘液性腫瘍(IPMN)の病理学的特徴を世界に先駆けて示し、また、ゲノム解析によりGNAS変異が特異的に高頻度に認められることを明らかにした。変異GNASをコンディショナルに発現できるマウスモデルを作成し、変異Krasと変異GNASを同時に膵特異的に発現させるとIPMNが発生することを示した。さらに、IPMNが浸潤がんに進展する過程や特徴的な組織型が発生する分子機序を明らかにした。
3. 外科切除された癌腫からオルガノイドを樹立し、ゲノム解析によって分子標的を同定し、標的薬剤の効果をオルガノイドで検証することで個別化治療を進められることを示した。

変異GNASの発現により発生したIPMN
Patients’ tumor-derived organoids
患者由来腫瘍組織オルガノイド
ARTICLE
Wang Y et al. Multi-omic profiling of intraductal papillary neoplasms of the pancreas reveals distinct patterns and potential markers of progression, Cancer Cell 2025.
URL:https://doi.org/10.1016/j.ccell.2025.08.001
Hirose K, et al. Clinicopathological relevance of SMAD4 and RUNX3 in patients with resected pancreatic cancer. Pancreas 54(4): e287-e294, 2025.
URL:https://doi.org/10.1097/MPA.0000000000002429
Suzuki S, et al. Three molecular developmental pathways of remnant pancreatic cancer after resection: A nationwide project study of Japan Pancreas Society. Ann Surg 281(6):1015-1025, 2025
URL:https://doi.org/10.1097/SLA.0000000000006444
Noguchi A, et al. Deep learning predicts the 1-year prognosis of pancreatic cancer patients using positive peritoneal washing cytology. Sci Rep 14: 17059, 2024.
URL:https://doi.org/10.1038/s41598-024-67757-5
Itoh T, et al. Gene rearrangement and expression of PRKACA and PRKACB governs morpho-biology of pancreatobiliary oncocytic neoplasms. Mod Pathol 37(1): 100358, 2024.
URL:https://doi.org/10.1016/j.modpat.2023.100358

 人工知能(AI)によって膵臓がん患者腹水中の生存関連因子を見つける ―腹腔内の免疫細胞が膵臓がん患者の生存期間に影響―
人工知能(AI)によって膵臓がん患者腹水中の生存関連因子を見つける ―腹腔内の免疫細胞が膵臓がん患者の生存期間に影響―
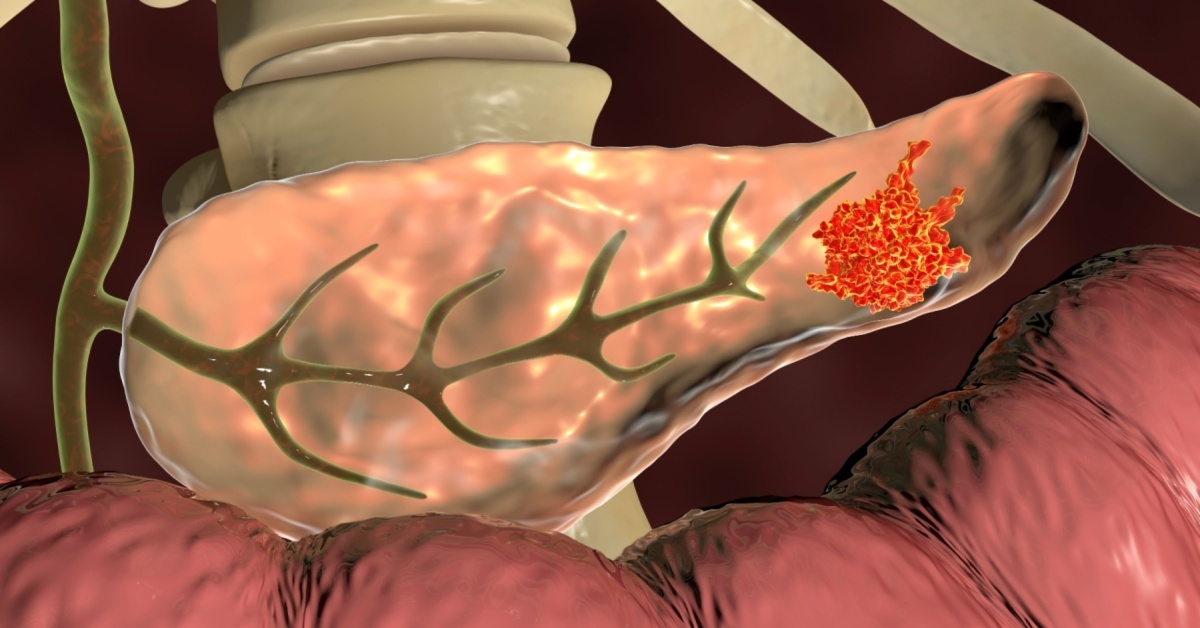 切除後の残膵がんにおける先行膵がんとの分子異常 プロファイル比較から残膵がん発生様式を解明 ~ 効果的な残膵がん診断治療戦略構築を目指して~
切除後の残膵がんにおける先行膵がんとの分子異常 プロファイル比較から残膵がん発生様式を解明 ~ 効果的な残膵がん診断治療戦略構築を目指して~
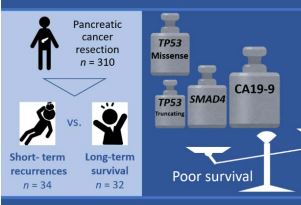 【研究成果】ゲノム解析を利用した膵癌予後予測~膵癌治療戦略への貢献に期待~
【研究成果】ゲノム解析を利用した膵癌予後予測~膵癌治療戦略への貢献に期待~
 【研究成果】全ゲノム解析等の網羅的ゲノム解析による消化器神経内分泌がんの病態解明~世界に先駆けて難敵ながんの本態を解き明し、薬剤開発の推進に期待~
【研究成果】全ゲノム解析等の網羅的ゲノム解析による消化器神経内分泌がんの病態解明~世界に先駆けて難敵ながんの本態を解き明し、薬剤開発の推進に期待~