Members
Mitsunori Fukuda (Cell biology)

Prof. Fukuda has graduated from Tohoku University, been given PhD thesis from the University of Tokyo, and now is a professor of Tohoku University, Graduate School of Life Sciences since 2006. He was the recipient of the 2004 Young Scientist Award from the Japanese Biochemical Society, the 2006 Young Scientist Award from the Kao Foundation for Arts and Sciences, and the 2007 Young Scientist Award from the Molecular Biology Society of Japan. He is currently a member of the editorial boards of two international journals, Calcium Binding Proteins and International Journal of Medical Engineering and Informatics. His research interest is to understand the mechanism of "membrane traffic.", in which membrane-wrapped substances (e.g., organelles) are transported within the cell, at the molecular level. More specifically, he is now focusing on the molecular mechanism of neurotransmitter release in neurons and melanin transport in melanocytes. He is a member of the Global COE project (2007-2011) supported by MEXT.
- Introduction of Research
-
The human body comprises a great many cells, each of which contains many subcellular units known as organelles (e.g., nucleus, the Golgi apparatus, and endoplasmic reticulum). Signals are exchanged frequently between cells and between organelles through ."membrane traffic.", in which membrane-wrapped substances are transported. However, much remains unknown about this process. Our laboratory focuses on the secretory phenomena (i.e., transport of secretory vesicles in neurons and endocrine cells) and on the melanin transport in melanocytes and tries to identify "key molecules" responsible for the secretion events and melanin transport. The aim of our research is to elucidate the molecular mechanism of membrane traffic by use of molecular biology, cell biology, biochemistry, and molecular imaging techniques.
We have previously shown that an abundant synaptic vesicle protein synaptotagmin I (Syt I) regulates synaptic vesicle exocytosis (i.e., neurotransmitter release) and endocytosis in neurons (Fig. 1). Syt I consists of a single N-terminal transmembrane domain and C-terminal tandem C2 calcium/phospholipid-binding domains (named C2A domain and C2B domain). These two C2 domains are functional domains of Syt I, because functionally blocking antibody against the C2A domain (or the C2B domain) inhibited synaptic vesicle fusion step (or synaptic vesicle recycling step) (Proc. Natl. Acad. Sci. USA (2000) 97, 14715-14719; Proc. Natl. Acad. Sci. USA (2004) 101, 17855-17860).
We have recently identified novel synaptotagmin-related molecules that contain tandem C2 domains at the C terminus (named Slp, synaptotagmin-like protein) and their related protein Slac2 (Slp homologue lacking C2 domains). Both Slp and Slac2 contain the conserved domain (named SHD, Slp homology domain) at their N terminus, and we found that the SHD functions as an effector domain for small GTPase Rab27A, which is specifically present on melanosomes in mammalian skin melanocytes (J. Biol. Chem. (2002) 277, 9212-9218). We further found that two Rab27A effectors, Slac2-a (also called melanophilin) and Slp2-a, are abundantly expressed on melanosomes and sequentially regulate melanosome transport in melanocytes (Nature Cell Biol. (2004) 6, 1195-1203). Slac2-a simultaneously interacts with Rab27A on the melanosome and with an actin-based motor myosin Va, and the resultant tripartite protein complex (Rab27A-Slac2-a-myosin Va) mediates actin-based melanosome transport (Mol. Cell. Biol. (2003) 23, 5245-5255). After actin-dependent melanosome transport, the second Rab27A effector Slp2-a promotes the anchoring of melanosomes to the plasma membrane of melanocytes through direct interaction of the C2A domain with phosphatidylserine (PS) (Fig. 2).
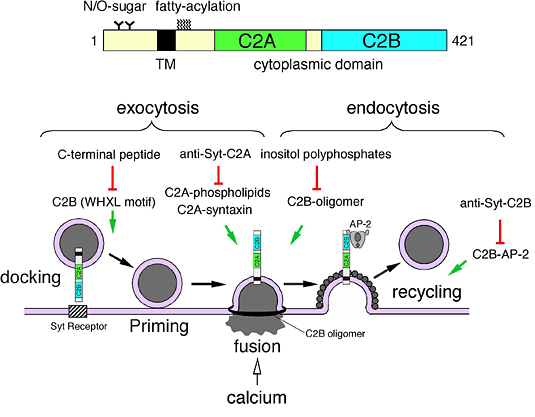
Fig. 1. Role of synaptotagmin I in synaptic vesicle transport in neurons (Molecular Mechanisms of Exocytosis (2006) pp. 42-61, Regazzi, R., ed., Landes Bioscience, Austin, TX).
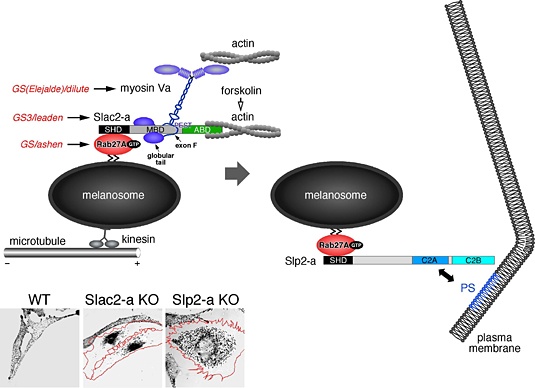
Fig. 2. Role of Rab27A and its effectors in melansome transport in melanocytes (J. Biochem. (2005) 137, 9-16).
- Articles
-
- Yu, E., Kanno, E., Choi, S., Sugimori, M., Moreira, J. E., Llinas, R. R. and Fukuda, M. (2008) Role of Rab27 in synaptic transmission at the squid giant synapse. Proc. Natl. Acad. Sci. USA 105, 16003-16008
- Tamura, K., Ohbayashi, N., Maruta, Y., Kanno, E., Itoh, T. and Fukuda, M. (2009) Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol. Biol. Cell 20, 2900-2908
- Kanno, E., Ishibashi, K., Kobayashi, H., Matsui, T., Ohbayashi, N. and Fukuda, M. (2010) Comprehensive screening for novel Rab-binding proteins by GST pull-down assay using 60 different mammalian Rabs. Traffic 11, 491-507
- Itoh, T., Kanno, E., Uemura, T., Waguri, S. and Fukuda, M. (2011) OATL1, a novel autophagosome-resident Rab33B-GAP, regulates autophagosomal maturation. J. Cell Biol. 192, 839-853
- Ohbayashi, N., Maruta, Y., Ishida, M. and Fukuda, M. (2012) Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J. Cell Sci. (doi:10.1242/jcs.094185)
- Mori, Y., Matsui, T., Furutani, Y., Yoshihara, Y. and Fukuda, M. (2012) Small GTPase Rab17 regulates the dendritic morphogenesis and postsynaptic development of hippocampal neurons. J. Biol. Chem. 287, 8963-8973