Members
Ichiro Sora (Biological Psychiatry)

Ichiro Sora, M.D., Ph.D. is Professor and Chairperson, Department of Biological Psychiatry, Tohoku University Graduate School of Medicine since 2002. Dr. Sora is also guest researcher at National Institute on Drug abuse, USA since 1999. He provides government service as members of advisory committees such as regulation of addictive drug, pharmaceutical and medical safety since 2006. Dr. Sora is trained as a psychiatrist (MD, 1982, PhD, 1986 Okayama University Medical School). His current research examines issues in molecular neurobiology of mental disorders. Dr. Sora is principle investigator of the major study of drug abuse, schizophrenia and attention deficit hyperactive disorder, funded by government grants from MHLW and MEXT.
- Introduction of Research
-
It is very useful to develop animal models of neuropsychiatric disorder, which are able to investigate pathophysiology, responsability to medication, and various symptoms of neuropsychiatiric disorder, although there are no sufficient animal models to reflect overall symptoms of psychiatric patients. Recently molecular genetic method, which targets specific genes of mice and delete them, has been established. We introduce here usefulness and recent acknowledgement of these knockout mice as animal models of neuropsychiatric disorder. 。。 Monoamine neurotransmission is affected by antipsychotics or antidepressants, so it is said to play a crucial role in pathophysiology of neuropsychiatric disorders. Dopamine transporter(DAT), serotonin transporter(SERT), and norepinephrine transporter are located on plasma membrane of pre-synaptic region. They rapidly reuptake extracellular monoamine and regulate the neurotransmission.
Drugs of abuse are able to elicit compulsive drug-seeking behaviors upon repeated administration,which ultimately leads to the phenomenon of addiction. Evidence indicates indicates that the susceptibility to develop addiction is influenced by sources of reinforcement, variable neuroadaptive mechanisms, and neurochemical changes that together lead to altered homeostasis of the brain reward system. Addiction is hypothesized to be a cycle of progressive dysregulation of the brain reward system that results in the compulsive use and loss of control over drug taking and the initiation of behaviors associated with drug seeking. Cocaine, amphetamine, and many other psychostimulants are believed to produce their stimulating and addictive effects mainly by increasing the extracellular levels of dopamine in the brain reward areas through the interaction with DAT. Mice lacking DAT are hyperactive and psychostimulants do not increase futher their locomotor activity. However, these mice still self-administer cocaine, as well as show conditioned place preference for cocaine, indicating that the drug's rewarding effects are not abolished. These data showed that although both DAT and SERT participate in cocaine's reward, DAT has more important role in this drug's effect. Studies in monoamine transporter knockout mice paint a picture of considerable complexity in the actions of cocaine.
Attention-deficit hyperactivity disorder。。(ADHD) is a clinically heterogeneous neuropsychiatric syndrome of inattention, hyperactivity, and impulsivity, typically of juvenile onset. We've engineered the dopamine tansporter knockout。。(DAT-KO) mice, which exhibit sponteneous behavioral hyperactivity compared to wild-type mice. Hyperactivity in DAT-KO mice is associated with high extracellular DA concentration because of lacking DA uptake by DAT. Recently, an association between polymorphisms in the noncoding regions of the human DAT and ADHD has been suggested. So DAT-KO mice has a possibility of animal model of ADHD. Treatment for ADHD has involved the use of psychostimulants that paradoxically serve to attenuate the hyperactivity and often improve cognitive performance. DAT-KO mice also showed a decrease in locomotion in response to amphetamine, methylphenidate, and cooaine. Despite the similarities between DAT-KO mice and ADHD, their phenotypes are not completely identical. It is not appropriate to explain the pathogenesis of ADHD with only monoamine transporters, other parameters that underlie the hyperactivity should be evaluated.
Exposure to drugs of abuse, such as amphetamine cause experience-dependent change in their behavioral effects. One example is 'behavioral sensitization', a process in which repeated intermittent administration of drugs causes increased responsiveness to their stimulant and rewarding effects. Repeated administration of the drug leads to increasing locomotor activity and stereotyped behavior in animal. Sensitization is a model of neural plasticity within which drug-induced changes in complex behavior can be linked to drug-induced changes in molecular processes.
Schizophrenia is one of the major psychiatric disorders, which shows psychotic symptoms of hallucination, delusion, and blunted affection. As most of antipsychotics for treatment of schizophrenia have potency as dopamine antagonists, "dopamine hypothesis" is well known to explain pathophysiology of schizophrenia.。。Prepulse inhibition (PPI) is reported to be disrupted and be one of the physiological characteristics in schizophrenics. PPI is a phenomenon in which the startle response is reduced when the startle stimulus is preceded by a low-intensity prepulse. Disruption in PPI is suggested to reflect the abnormality of sensorimotor gating. Recently PPI paradigm has been used as one of the measurements in developed animal models of schizophrenia. For example, drug treatment of direct and indirect dopamine agonists or DATKO mice, which significantly increases dopaminergic neurotransmission, is disrupted in PPI.
Opiate analgesics are widely used and abused drugs.。。Individual differences in opiate sensitivity can hamper effective pain treatments and increase risks of drug abuse. Although genetic factors might affect individual differences in opiate sensitivity, scientific evidence for specific genetic mechanisms that underlie these differences has been sparse. Recent studies using inbred and knockout mice have revealed that the mu opioid receptor (MOR) gene has a mandatory role in the analgesic and addictive properties of opiate drugs. More than 100 polymorphisms have been identified in the human MOR gene, some of which are related to vulnerability to drug dependence in some populations. Rapid advances in this research field are leading to improved understanding of the relationships between gene polymorphisms and opiate sensitivities that will enable more-accurate prediction of the opiate sensitivity and opiate requirements in individual patients.
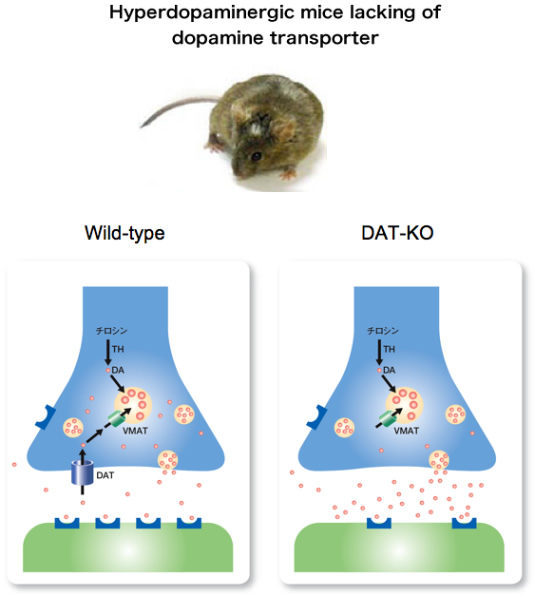
Figure 1.
The dopamine transporter (DAT) functions to limit extracellular dopamine through reuptake of released dopamine. In mutant mice lacking the DAT, extracellular dopamine levels are persistently elevated, which induces hyperdopaminergic neurotransmission.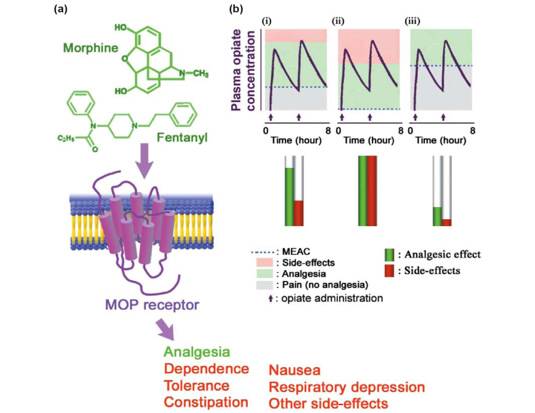
(a) Opiate analgesics, such as morphine and fentanyl, act mainly on mu opioid receptor (MOR) and induce analgesia together with adverse side-effects such as dependence, tolerance, constipation, nausea and respiratory depression. (b) Opiate sensitivity differs among individuals at least partly because of the differences in the MOR gene. Although the plasma opiate concentration after its administration (arrows) changes in the same manner in different patients (i-iii), the clinical response can differ between patients because of 5-10-fold inter-patient differences in the minimal effective analgesic concentration (MEAC) (dashed blue line).
- Articles
-
- Uhl GR, Li S, Takahashi N, Itokawa K, Lin Z, Hazama M, Sora I. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J 14: 2459-2465 (2000)
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA 98: 5300-5305 (2001)
- Ikeda K, Ide S, Han W, Hayashida M, Uhl GR, Sora I. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol Sci 26(6): 311-317 (2005)
- Ide S, Kobayashi H, Ujike H, Ozaki N, Sekine Y, Inada T, Harano M, Komiyama T, Yamada M, Iyo M, Iwata N, Tanaka K,Shen H, Iwahashi K, Itokawa M, Minami M, Satoh M, Ikeda K, Sora I. Linkage disequilibrium and association with methamphetamine dependence/psychosis of mu-opioid receptor gene polymorphisms. Pharmacogenomics J 6(3):179-188 (2006)
- Yamashita M, Fukushima S, Shen H, Hall FS, Uhl GR, Numachi Y, Kobayashi H, Sora I. Norepinephrine Transporter Blockade Can Normalize the Prepulse Inhibition Deficits Found in Dopamine Transporter Knockout Mice. Neuropsychopharmacology 31(10):2132-2139. (2006)